-

2016 Halawai Makahiki
Ua mālama ʻo Shuangyang Medical i kahi pāʻina hālāwai makahiki ma Ianuali 18, 2017 e hoʻomaikaʻi aku i nā limahana a pau no kā lākou hana paʻakikī ma 2016, a makemake i nā hoa hana i ke olakino maikaʻi, hauʻoli ʻohana a me ka hana me nā mea a pau i ka makahiki hou! ...Heluhelu hou aku -

2016 COA Hōʻike
ʻO ka 18th orthopedic academic conference a me ka 11th COA international academic conference ma 2016 i mālama ʻia ma Beijing National Conference Center mai Nowemapa 17, 2016 a hiki i Nowemapa 20, 2016. Ke kali nei e hālāwai me ʻoe ma ke keʻena Lapaʻau Shuangyang. ...Heluhelu hou aku -

2014 COA Hōʻike
ʻO ka 16th Chinese Orthopedic academic conference a me ka 9th Chinese Orthopedic Association (COA) e mālamaʻia ma Beijing National Conference Center mai Nowemapa 20 a hiki i 23, 2014. Ke kali nei e hālāwai meʻoe ma ke keʻena Lapaʻau Shuangyang. ...Heluhelu hou aku -

Nā Palapala Sila Nui Hoʻolālā
Ua hoʻokuʻu ʻia ka ʻōnaehana anatomic titanium mesh a me ka titanium binding system, a ua loaʻa iā mākou nā palapala patent design no nā huahana 25.Heluhelu hou aku -

Lokcing Maxillofaical Pūnaehana Hoʻopaʻa Kūloko
Ua hoʻokuʻu ʻia ka ʻōnaehana hoʻopaʻa i loko o ka maxillofacial a me ka ʻōnaehana hoʻopaʻa paʻa iwi paʻa.Heluhelu hou aku -
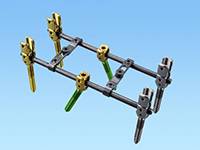
Hoʻomohala Huahana Hou
Hoʻomohala i ka ʻōnaehana koʻokoʻo spinal spinal, laka i ka hale fusion hui, ʻōnaehana nail interlocking intramedullary metala, nā huahana lāʻau lapaʻau haʻuki, a me nā kākoʻo hoʻoponopono waho.Heluhelu hou aku -

Pūnaehana hoʻokele maikaʻi
Hoʻomaikaʻi mau i ka ʻōnaehana hoʻokele maikaʻi e like me ka Good Manufacturing Practice for Medical Devices (Trial) a me ka hoʻoponopono hoʻokō no nā mea lapaʻau Implantable o ka hana hana hana maikaʻi no nā mea lapaʻau (Trial)Heluhelu hou aku -

Hanohano ka Mea Hana Mea Hana Lapaʻau Credible
Ua helu ʻia mākou ʻo Credible Medical Device Manufacturer o Jiangsu.Heluhelu hou aku -

Hoʻoholo i ka nānā ʻana o ka National Bureau
Ua lilo mākou i mea mua e hoʻoholo i ka nānā ʻana e like me ka Enforcement Regulation (Pilot) for Implantable Medical Devices of Good Manufacturing Practice for Medical Devices i hoʻonohonoho ʻia e ka National Bureau.Heluhelu hou aku -

palapala hōʻoia CE
Loaʻa iā mākou ka palapala CE i hāʻawi ʻia e TUV.Heluhelu hou aku -

ʻOihana ʻepekema pilikino a me ka ʻenehana
Ua hala mākou i ka hōʻoia ʻana o ka ʻōnaehana hoʻokele maikaʻi o ka lāʻau lapaʻau o CMD, a helu ʻia ma ke ʻano he Private Scientific and Technological Enterprise o Jiangsu.Heluhelu hou aku -

Palapala ISO
Ua hala ISO9001: 2008 Quality Management System Certificate. Ua hala ISO13485: 2003 Lapaʻau Manaʻo Manaʻo Manaʻo Manaʻo Pūnaewele. Ua lanakila 'o ia i ka makana o Kaulana & Kona Huahana ma Suzhou.Heluhelu hou aku